Trust and know-how cultivated through experience
-
2,000+ projects, 500+ companies
We provide full support for manual production with know-how gained from production experience of more than 2,000 projects for over 500 companies.
-
Extensive track record in medical devices and industrial machinery
We have extensive experience in producing manuals in a wide range of fields, especially for industrial machinery and medical devices. Our experts in those fields will provide full support for production of manuals by utilizing their know-how.
Pursuing customer satisfaction
-

-
Outsourcing manual production
Customer success has to be achieved from various aspects when producing manuals including usability, which is an important quality element of manuals, risk reduction related to product safety, compliance with laws and regulations and support for globalization, and digitalization. Feel free to contact us if you have any concerns about manual production. Our specialists are available to provide outsourcing services for manual production.
-
Improving customer satisfaction for your products
We seek to improve customer satisfaction in terms of product usability and the usability of the manual, such as ""easy-to-understand"" and ""easy-to-search"" by participating in the development process from the product planning stage, according to your request.
-
-
Producing manuals achieving their purpose
We provide production services to achieve the fundamental purpose of the manual, not bounded by design of the look and feel or de facto standards.
We produce manuals not only to convey accurate and easy-to-understand information but also to enhance the product's value by considering the manual as a part of the product.
In the planning phase, we will research and analyze the materials (specifications, existing manuals, etc.) provided and the necessary legal regulations and standards and propose a separate volume structure, table of contents structure, sample writing and sample design to meet the requirements. We also provide the following services upon request.- Conduct risk assessment from the user's perspective and incorporate the results in the manual to reduce risk
- Integrate verification of effectiveness into the production process to ensure that the manual is useful
-
Visualizing the true quality of manuals
Using our unique system, "ManualDock", we can help you visualize the true quality of manuals and propose significant improvements in quality. "ManualDock" evaluates your manual from the following perspectives and makes suggestions for improvement.
- Appropriateness (usefulness)
- Accuracy (correctness)
- Ease of understanding (written expression)
- Ease of viewing (design)
- Ease of search (searchability)
- Safety (user protection)
Click here for more information on manual doc.

Compliance support

-
Enhancement of compliance support
We provide compliance services in relation to the Product Liability Law (PL Law) for the destination country of your products by collaborating with regulatory experts to ensure that your manuals comply with the PL Law (ANSI and other standards), CE marking and SEMI regulations. We can conduct a risk assessment of products from the perspective of manual users upon request, send feedback of the risks identified to safety and protection design, and also assist in reflecting residual risks not covered by design in manuals and labels.
-
Comparative analysis
We will objectively evaluate the usability and compliance with legal and standard requirements by comparing and analyzing the manuals of your product with the manuals of competitors. You can improve your manuals based on the results of the evaluation and make them stand out by producing manuals that are superior to those of other companies in the industry.
Cost performance
-
Reducing load on call centers
By producing easy-to-understand manuals with high searchability based on information such as FAQs, you can reduce the number of customer inquiries and significantly reduce the workload of call centers, sales, support and training departments.
-
Effective use of existing software assets
We can effectively use software assets and study ways to improve efficiency by analyzing the existing manuals. The software assets for translation can be effectively used in the same way for English, Japanese and multilingual manuals.
-
Development and effective use of writing standards
By preparing writing standards, the writing style will be unified to establish a corporate identity for manual production, including design.
-
Centralized management through modularization
If a product lineup has multiple similar products, we will modularize the information in groups (e.g. by chapters) to separate common and unique information. Significant cost savings can be realized when preparing manuals of a new product by combining the modules.
-
Cost reduction through multiple deployments using single source
We produce online manuals such as web manuals and HTML Help. By utilizing WebWorks ePublisher and RoboHelp, one single source can be effectively used for multiple purposes.

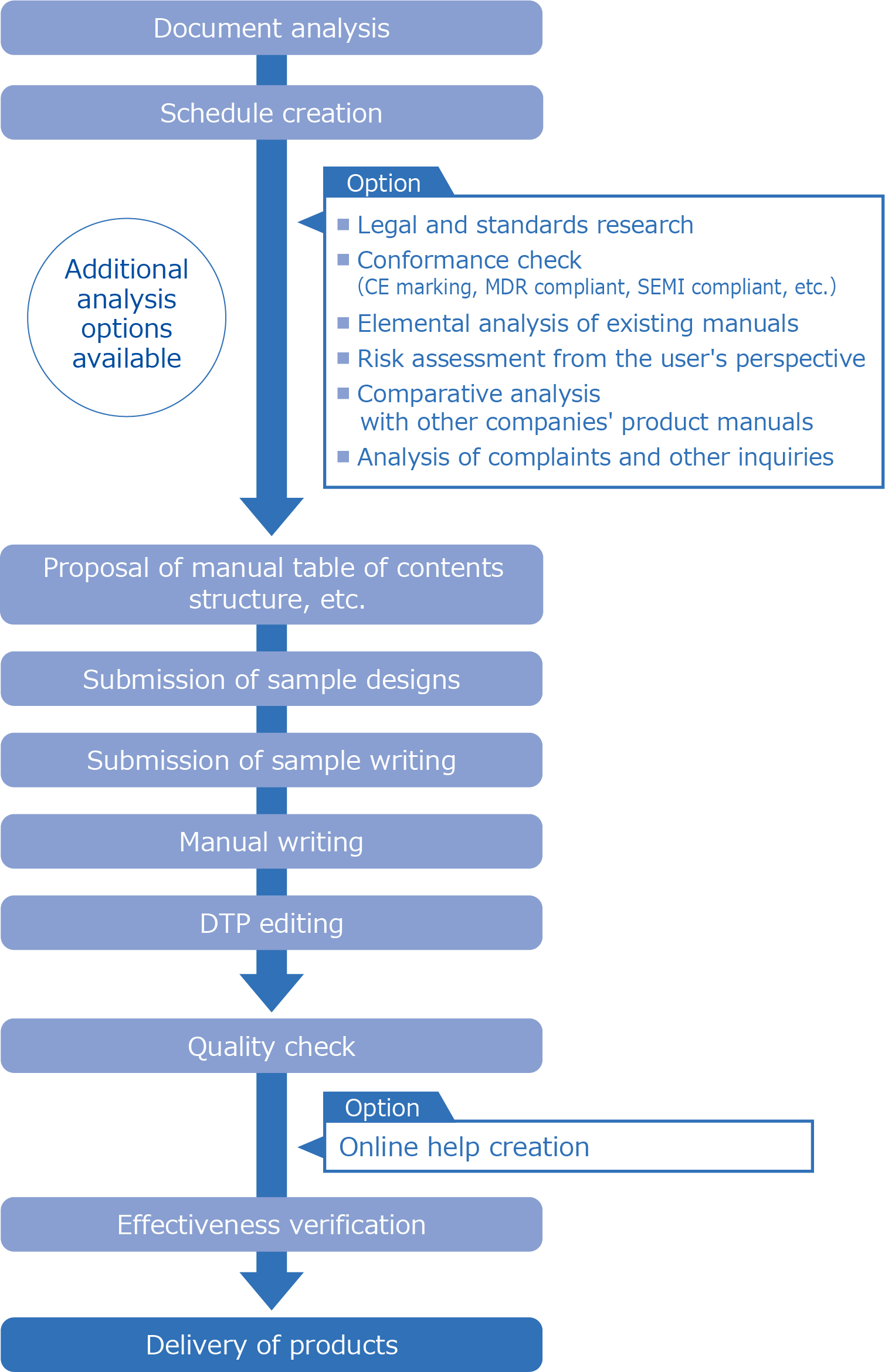
Manual production flow
We produce manuals using the following work process.
We offer many options, including research on/checking conformity with laws and regulations and standards related to target products, analysis of the elements to be included in the manual, and analysis for manual improvement. Please feel free to contact us.
Various services
-
Manual Production
Medical Device Instruction Manual (IFU) Production

We support creating various instruction manuals for medical devices (Instruction for Use (IFU) manuals for health care providers, manuals for patients, maintenance manuals, technical documents, package inserts and other various files). We can also provide translation and DTP editing for medical terminology, and create labels that comply with safety standards.
Based on our experience in supporting a wide range of requests from major medical device manufacturers, we can provide a one-stop service that includes not only IFU creation, but also multilingual development, including English translation. -
Manual Production
Industrial Machinery Manuals Production

A team of professionals specializing in B2B product manuals will take charge of producing your manual. For operation manuals, safety manuals, maintenance manuals and installation manuals, etc., we will focus on its searchability and produce manuals that considers human errors.
Examples
of supported
manuals- Operations manual
- Maintenance manual
- Safety manual
- Installation manual
- Parts list (for creating technical reference)
-
Manual Production
End-user Manual Production

"We produce manuals with excellent usability emphasizing on easy-to-understand expressions and ease of use.
By reflecting the analysis results of FAQ and call center inquiry, we will produce manuals with excellent customer satisfaction. This will reduce the load on call centers.
Verifying the effectiveness of the manual from the user's perspective is extremely important when producing manuals for end-users.
Effectiveness verification includes both usability verification and risk assessment from the user's perspective." -
Manual Production
Business Manual Production

We create easy-to-understand work manuals that can also be used for in-house training by incorporating many visual expressions such as marks and symbols with a structure focusing on the business flow chart.
Medical Device Instruction Manual (IFU) Production

We support creating various instruction manuals for medical devices (Instruction for Use (IFU) manuals for health care providers, manuals for patients, maintenance manuals, technical documents, package inserts and other various files). We can also provide translation and DTP editing for medical terminology, and create labels that comply with safety standards.
Based on our experience in supporting a wide range of requests from major medical device manufacturers, we can provide a one-stop service that includes not only IFU creation, but also multilingual development, including English translation.
-
Laws/regulations/standards compliance
Regulatory investigation
We conduct research on laws and regulations concerning manuals and labels when exporting medical devices from Japan. We have partnered with certification bodies and specialized research firms that have regulatory expertise, to conduct research on laws and regulations in the country to which products are shipped.
Incorporating evaluation reports from testing and certification bodies
When you request a third-party testing and certification organization for safety certification of your product, they will issue an assessment report showing if the various manuals and warning labels that are part of the product comply with the applicable laws and regulations and standards. We will work on your behalf in accordance with the evaluation report to ensure that your manuals comply with the requirements.
Laws/regulations/standards compliance evaluation
We provide services for the evaluation of compliance with standards in the medical field, including the IEC 60601-1 series. Upon request, we will study and analyze the applicable regulations in the destination countries or in each medical field and support you in reflecting the results in the IFU.
We also provide support for reflecting the new requirements in the IFUs in accordance with the transition of the EU (European Union) regulation on medical devices from Medical Device Directive (MDD) to the European Medical Device Regulation (MDR).
See the Document Safety website for more information on the transition from MDD to MDR. -
Risk assessment of medical devices (user perspective)
We deal with a wide range of medical devices in medical field, ranging from home medical devices (blood pressure monitors, etc.) to medical devices that require special knowledge and a higher level of safety, such as endoscopes and artificial hearts.
We support the risk management that is required for medical devices. We conduct risk assessment and risk analysis from the user's perspective and report the analysis results. We will provide feedback on residual risks to the design department for improving safety and protection and reflect them in the manuals and labels. -
IFU comparative analysis with competitors' medical devices
We will objectively evaluate the usability and compliance status with laws and standards by comparing and analyzing the IFUs of your product with that of your competitors' products. You can improve your IFUs based on the results of the evaluation and make them stand out by producing IFUs that are superior to those of competitors' in the industry.
-
Medical translation (including MDR)
We provide high quality translations by translators having expertise in the medical field. Our experts can handle Japanese, English and multilingual translation.
We can also support you in preparing English versions of documents newly required in the MDR (European Medical Device Regulation). For such translations, we ensure appropriate medical translations by using a database of general standards for medical devices and common names for medical devices (JMDN) that we have compiled. We can also create a database of individual standards specific to the field of your product, so do not hesitate to contact us. We also support DTP editing for multilingual translation.
See the Document Safety website for more information on the MDR support.
-
Technical translation
High quality translations through the experience in creating IFUs and package inserts for medical devices
Our translators specialize in medical devices and can translate a wide range of medical documents for pharmaceutical and medical device companies. Since the top priority in the translation of medical and pharmaceutical documents is accuracy, we have established a thorough quality check system by native speakers. We will provide translations taking into account the terminology for regulatory information (laws and regulations and standards, etc.) related to your medical devices. We also provide full support for Japanese companies entering the overseas market and for foreign companies entering the Japanese market.
Industrial Machinery Manuals Production

A team of professionals specializing in B2B product manuals will take charge of producing your manual. For operation manuals, safety manuals, maintenance manuals and installation manuals, etc., we will focus on its searchability and produce manuals that considers human errors.
of supported
manuals
- Operations manual
- Maintenance manual
- Safety manual
- Installation manual
- Parts list (for creating technical reference)
-
Compliance with laws and regulations and standards
Regulatory investigation
We conduct research on laws and regulations concerning manuals and labels when exporting industrial machinery from Japan. We have partnered with certification bodies and specialized research firms that have regulatory expertise, to conduct research on laws and regulations in the country to which products are shipped.
Incorporating evaluation reports from testing and certification bodies
When you request a third-party testing and certification organization for safety certification of your product, they will issue an assessment report showing if the various manuals and warning labels that are part of the product comply with the applicable laws and regulations and standards. We will work on your behalf in accordance with the evaluation report to ensure that your manuals comply with the requirements.
Laws/regulations/standards compliance evaluation
We evaluate the conformance of product manuals to various safety standards (IEC60204-1, IEC60601-1, ISO12100, SEMI S2, SEMI S8 and SEMI S13, etc.) and incorporate the results in manuals and labels. If necessary, we also evaluate compliance status of existing product manuals with various laws and regulations (New Machinery Directive, etc.), and with various safety standards.
-
Risk assessment of industrial machinery (user perspective)
By assessing the risk of potential nature of danger (mechanical, electrical, thermal, chemical, etc.) and foreseeable misuse in each process of a product life cycle, we will propose risk assessment and risk reduction as prescribed by international safety standards such as ISO12100 and ISO14971.
-
Comparative analysis
We will objectively evaluate the product safety and compliance status with laws and standards by comparing and analyzing the manuals of your company with the manuals of your competitors' products. You can improve your manuals based on the results of the evaluation and make them stand out by producing manuals that are superior to those of other companies in the industry.
-
Technical translation
High-quality translations through the experience in creating various manuals of industrial machinery
We provide high-quality translation services specializing in industrial machinery, leveraging our translation experience in the industrial machinery field. We can also provide multilingual translation and DTP editing in compliance with laws and standards for industrial machinery.
End-user Manual Production

We produce manuals with excellent usability emphasizing on easy-to-understand expressions and ease of use.
By reflecting the analysis results of FAQ and call center inquiry, we will produce manuals with excellent customer satisfaction. This will reduce the load on call centers.
Verifying the effectiveness of the manual from the user's perspective is extremely important when producing manuals for end-users.
Effectiveness verification includes both usability verification and risk assessment from the user's perspective.
-
Call Center Data Analysis
Inquiries to the call center or sales department are where the real voices of users, such as complaints and various questions, are collected. We will identify the usability problems of the product, shortcomings of the manual, and potential risks by analyzing the data and propose improvements.
-
Manual analysis
Identifying and analyzing problems
If you provide us with a manual or document that you want to improve, we will study the appropriate requirements of those and identify the missing elements to achieve their purpose. We will investigate not only the literary style but also the structure of the table of contents and the visual aspects such as illustrations and layout, and propose specific measures with samples of the improvements.
Comparative analysis
We will objectively evaluate the usability and legal and standard requirements by comparing and analyzing the manuals of your product with those of competitors' products provided. You can improve your manuals based on the results of the evaluation and make them stand out by producing manuals that are superior to those of other companies in the industry.
-
Effectiveness Verification
We offer various tests to verify whether the contents of manuals and video manuals, etc., are useful and suited for the intended purpose, while operating the actual product. We can also examine your existing contents produced elsewhere.
Usability verification
We will verify multiple items from different aspects from the user's perspective, such as whether the content is easy for the target user to understand and whether the users can achieve their goal without stress. If you want to verify the usability of the entire product, we can perform the verification by applying our elemental analysis to the user interface of the product and the manual.
Operation verification
We will operate the target products and software using the product manuals and online help to verify whether the described information is correct and whether the expressions are appropriate.
-
Product risk assessment (user's perspective)
We will identify any risks and problems associated with the product by conducting risk assessment, effectiveness verification and usability check from the user's perspective in line with the product life cycle. We will submit proposals given on the right for the identified risks to lower the risk.
- Additions or changes to the safety design of the product
- Adopting protective designs and reviewing support systems
- Improvement of manuals, additions or changes to warning labels, etc.
-
Technical translation
High-quality translations through the experience of producing instruction manuals for consumer electronics
Based on our translation experience in the field of consumer electronics, we will provide bilingual translations not only converting one language to another, but including an awareness of the ""differences"" in ideas and values based on history, culture, etc. We can also provide multilingual translation and DTP editing in compliance with laws and standards of the consumer electronics field.
Business Manual Production

We create easy-to-understand work manuals that can also be used for in-house training by incorporating many visual expressions such as marks and symbols with a structure focusing on the business flow chart.
-
Business efficiency consultation
By assisting you from the process analysis stage of your current operations, we can help you to not only create operation flows but also review them and identify risks. We can propose a operations manual that is specific to your system.
-
Benefits of creating operations manuals
Preparing an operations manual ensures that anyone can execute the work and the manual will also serve as a bible during personnel changes or when emergency response is required.
Provide stable services and operations
The manual helps unify work methods without relying on the skills of each personnel. This will ensure providing services and operations at a certain quality level.
Operation reviews
By conducting operation analysis during the creation stage of the operations manual, you can identify the operations to improve. We not only eliminate unreasonable, pointless and inconsistent work but also identify hidden risks in the operations at the process analysis stage.
Building common assets
Information sharing and skill transfers can be made easier by making individual assets such as techniques and know-how tangible.
Application to new employee training
Training costs can be reduced and training period shortened by using operation manuals that are based on actual business practices for training new employees.
Clarification of responsibility and authority
Preparing operation manuals clarifies the role and code of conduct of each employee, leading to higher levels of operations and service quality.


